COVID-19 vaccines explained: a comparison with veterinary vaccines shows what’s really new and not so new
Never before in their 225-year history have vaccines been so relevant and important to the health and wellbeing of the global population.

The last year has witnessed unparalleled efforts by scientists and clinicians around the world to develop, test and deploy vaccines against Covid-19.
These efforts have led to remarkable achievements: vaccines that have now become household names are being rolled out at huge scale less than a year after the disease was first diagnosed.

These vaccines span the spectrum of vaccine types, ranging from the conventional, such as live attenuated whole virus and inactivated whole virus, to the more innovative vaccine technologies, such as sub-unit, viral vector and RNA vaccines.

Below, I will try to explain the basic features and benefits of the different types of vaccine and give some examples of Covid-19 vaccines and other types of human and animal vaccines, including Ceva’s own vaccines that fall into each type.

Types of Vaccine

An “obvious” way to make a vaccine is to take the disease-carrying virus or bacterium, or one very similar to it, and inactivate or kill it using chemicals, heat or radiation. This approach uses technology that’s been proven to work in people and vaccines can be manufactured on a reasonable scale.
However, it requires special laboratory facilities to grow the virus or bacterium safely, can have a relatively long production time, and depending on the type of antigen it will likely require one or two doses to be administered.

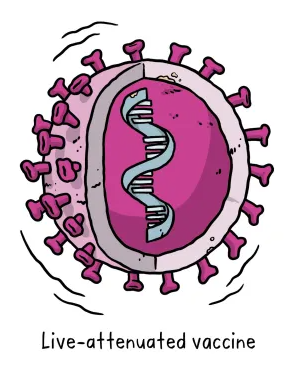
A live-attenuated vaccine uses a living but weakened version of the virus or one that’s very similar. This approach uses technology that’s been proven to work in people and animals and vaccines can be manufactured on a reasonable scale. However, vaccines like this may not be suitable for people with compromised immune systems, and the safety in general have to be carefully assessed.

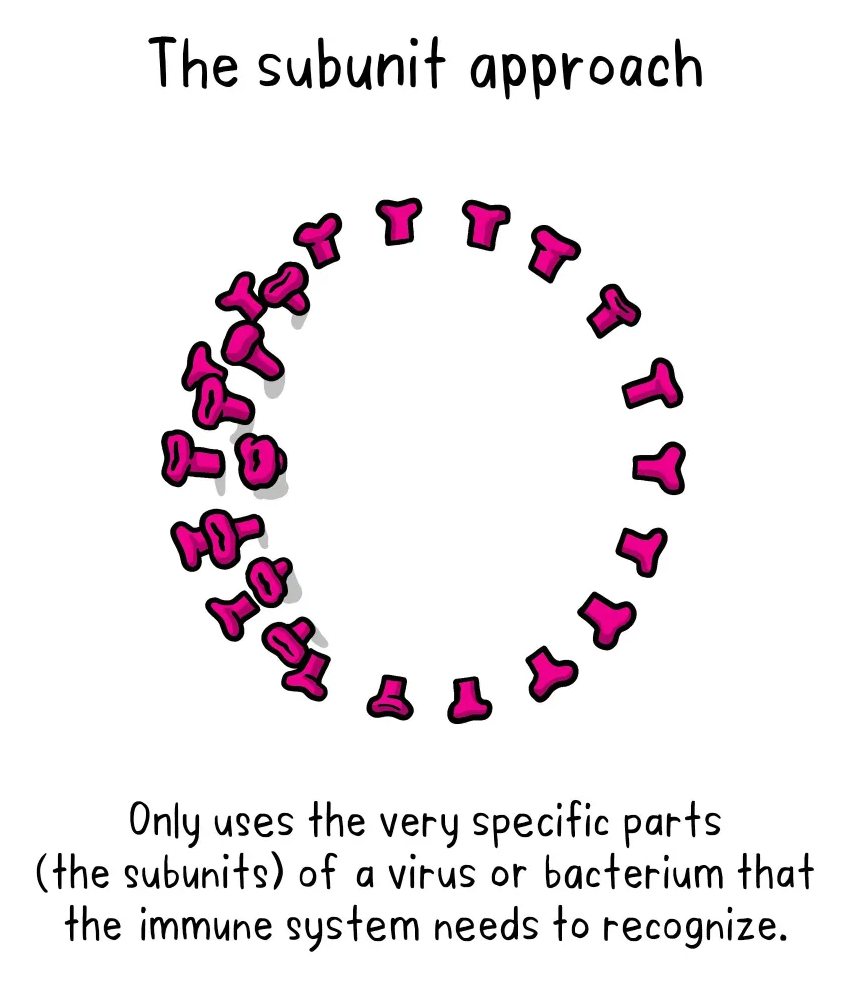
A subunit vaccine is one that only uses the very specific parts (the subunits) of a virus or bacterium that the immune system needs to recognize. It doesn’t contain the whole microbe. The subunits are typically peptides or proteins, maybe sugars.

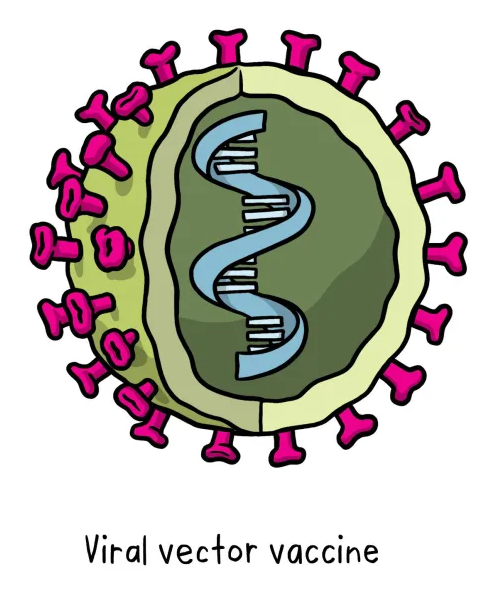
This type of vaccine uses a safe virus as a vector to deliver the genetic information for a specific sub-parts – called proteins, or antigens – of the germ of interest so that it can trigger an immune response without causing disease. To do this, the genetic instructions for making particular parts of the pathogen of interest are inserted into a safe viral vector. The safe virus then serves as a platform or vector to deliver this information into the body, where the specific proteins (antigens) are subsequently produced. These proteins then trigger the immune response. This type of vaccine can be developed and updated rapidly, once the vector system based on a safe virus has been already established (this requires extended research).

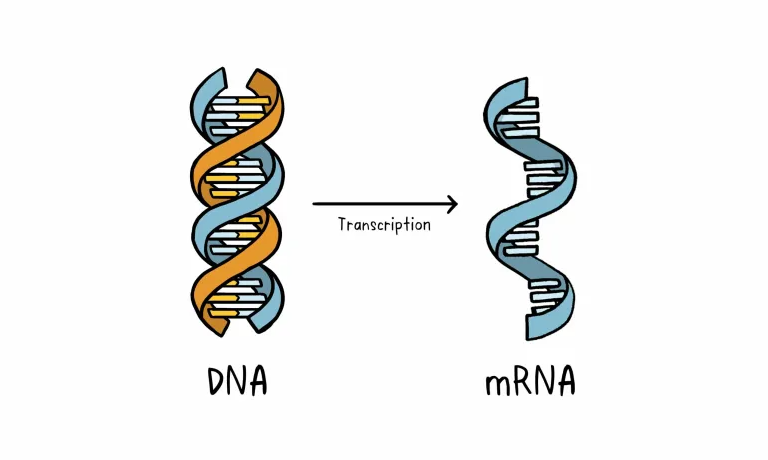
Unlike vaccine approaches that use either a weakened or dead whole microbe or parts of one, a nucleic acid vaccine just uses a section of genetic material that provides the instructions for specific proteins, not the whole microbe. DNA and RNA are the instructions our cells use to make proteins. In our cells, DNA is first turned into messenger RNA, which is then used as the blueprint to make specific proteins.
A nucleic acid vaccine delivers a specific set of instructions to our cells, either as DNA or mRNA, for them to make the specific protein that we want our immune system to recognize and respond to.
The nucleic acid approach is a new way of developing vaccines. Before the Covid-19 pandemic, none had yet been through the full approvals process for use in humans, though some DNA vaccines, including for particular cancers, were undergoing human trials. Because of the pandemic, research in this area has progressed very fast and some mRNA vaccines for Covid-19 are getting emergency use authorization, which means they can now be given to people beyond using them only in clinical trials.

People often ask, what is the best vaccine against Covid-19. The answer is simple – the one that is available to you! All licensed vaccines are safe and have good efficacy, especially against severe illness and death.
Zoltan Penzes – Head of Biology R&D, Ceva
